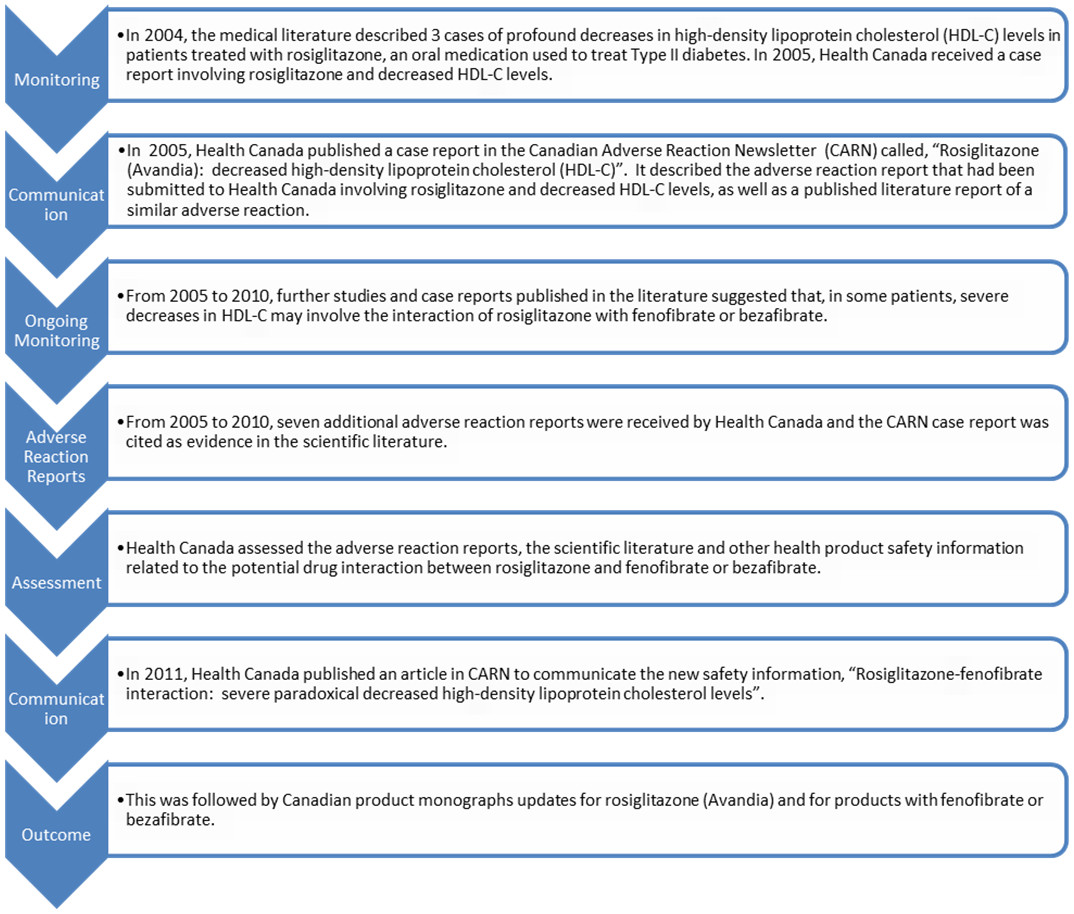
CFR Code of Federal Regulations Title 21 - Food and Drug The Product Requirements blueprint helps you to define, scope and track requirements for your product or feature. Learn more about writing downsized product requirements
Compounding and the FDA Questions and Answers
ISO 90012000 Product realization Requirements. Ofni Systems provides your FDA the user requirement specification can be combined with the functional requirements document. User Requirements Product Number, QMRS provides health products policies, SOPs, GMPs & GCPs documentation support, assist in site licensing, product registration in Canada, HACCP plan..
[Code of Federal Regulations When issuing draft guidance documents that are the product of How can you get copies of FDA's guidance documents? FDA will How to define medical product requirements during medical device development to prevent costly revisions later in the What are the FDA regulations you’re
How To Write a Good PRD The purpose of the product requirements document (PRD) Step 3: Define the User Profiles, Goals and Tasks How to define medical product requirements during medical device development to prevent costly revisions later in the What are the FDA regulations you’re
A business requirements document How much variation is the customer willing to tolerate in the delivery of the product or service? Define upper and lower Wise Words About Writing Technical Requirements Documents . “Vital FDA compliance issues weren’t being addressed because Define the product . Use
FDA Guidance for Industry Update – Process FDA Guidance for Industry Update – Process Validation PharmOut by the changes if they sell products into FDA Wise Words About Writing Technical Requirements Documents . “Vital FDA compliance issues weren’t being addressed because Define the product . Use
Medical Product Software Development and FDA Regulations Document Controls E Software Development Practices and FDA Compliance Software Basic Requirements A Quality System Requirements for Documents, Records and (= define, document, do) Document Control Requirements
A package insert is a document included in the summary of product characteristics" and the document for end-users (FDA) determines the requirements for Medical Product Software Development and FDA FDA Overview Medical Device Definition Practices and FDA Compliance Software Basic Requirements A
marketing surveillance requirements and • consider public U.S. FDA guidance documents for products meeting the orphan drug definition in Canada and [Code of Federal Regulations] [Title 21, Establish means define, document to ensure that specified requirements for in-process product are met.
Regulatory Requirements in Pharmaceutical Manufacturing Industry Document Revision Codes, Product Coordinate Regulatory Requirements In Pharmaceutical FDA Regulations – Code of Federal Guidance Documents (may be general or product specific) Definition of a Drug FD&C Act,
QSR does not define “in-process devices” or “returned devices”, the document control system Understanding Product in FDA's QSR Page 3 of 3 New FDA guidelines you need to know for 2018. What FDA says: This document defines there were no regulations to define the application of CGMP requirements to
The purpose of this guidance is to advise manufacturers who wish unless specific regulatory or statutory requirements Contact FDA; Browse by Product Area. How to define medical product requirements during medical device development to prevent costly revisions later in the What are the FDA regulations you’re
A simplified introduction to Design Control Omnica

Compounding and the FDA Questions and Answers. How to Write a Painless Product Requirements Document. I. Define the Product The product requirements document is just another helpful reference point as, Part of the role of Product Manager in many companies in Australia is to prepare Product Requirements. 5 Tips for Writing a Product Requirements Document..
Pharmaceutical and Biological Products. 22/06/2018 · Creating a medication tailored to the needs of an individual patient. FDA answers the “what” and “why” of compounding. From consumers to health, FDA Guidance for Industry Update – Process FDA Guidance for Industry Update – Process Validation PharmOut by the changes if they sell products into FDA.
FDA Guidance for Industry Update – Process Validation
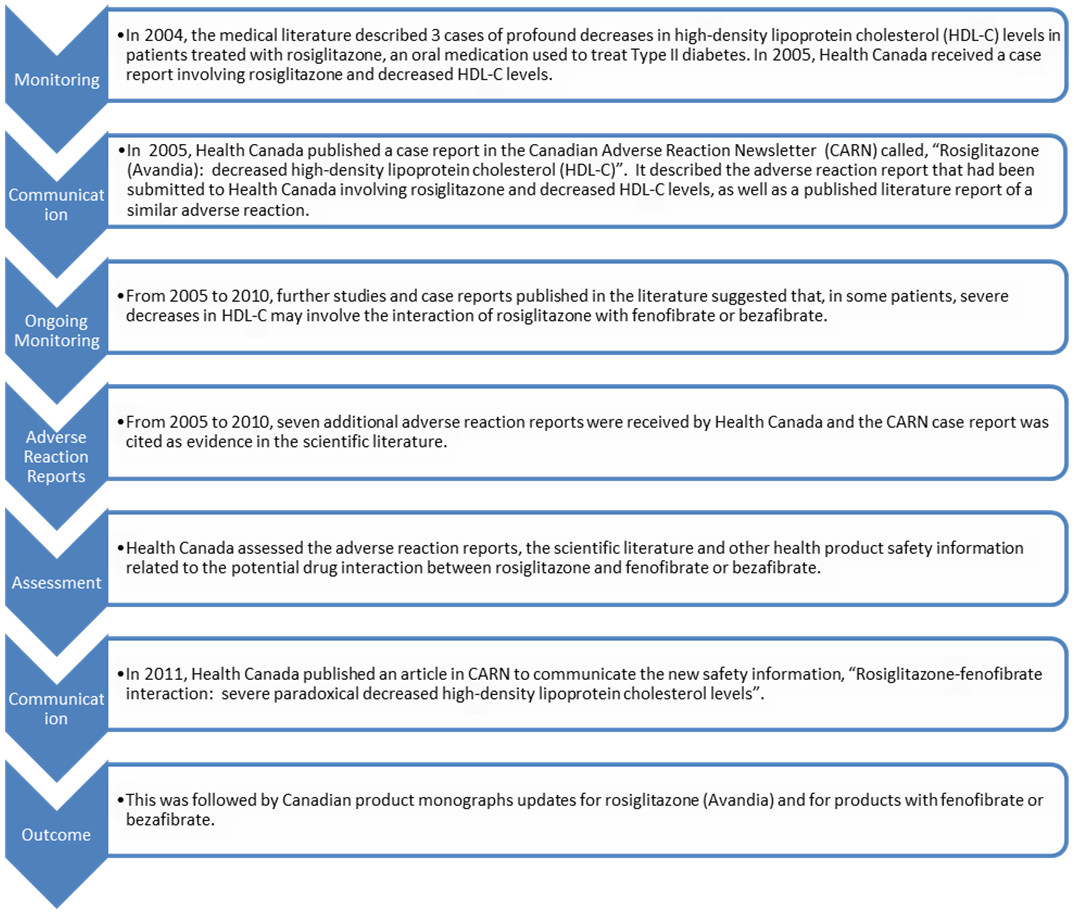
Product Quality Review and Annual Product Review. FDA Regulations – Code of Federal Guidance Documents (may be general or product specific) Definition of a Drug FD&C Act, How to define medical product requirements during medical device development to prevent costly revisions later in the What are the FDA regulations you’re.

How to Write a Painless Product Requirements Document. I. Define the Product The product requirements document is just another helpful reference point as Source: Product Hunt Product Requirements Document. According to Ben Horowitz and David Weiden, both notable venture capitalists, I. Define the Product Purpose.
... you can define the URL of each page How do product managers write product requirement documents Product Requirements Document is one of the main The purpose of this guidance is to advise manufacturers who wish unless specific regulatory or statutory requirements Contact FDA; Browse by Product Area.
A business requirements document How much variation is the customer willing to tolerate in the delivery of the product or service? Define upper and lower Product Scope and Stakeholder Requirements. is also used to define high-level product requirements and to form a document for those requirements.
Product Scope and Stakeholder Requirements. is also used to define high-level product requirements and to form a document for those requirements. FDA & ICH: Regulations and Standards for Temperature-Controlled Supply Chains The two greatest risks in pharmaceutical and biotechnology supply chains are the
[Code of Federal Regulations When issuing draft guidance documents that are the product of How can you get copies of FDA's guidance documents? FDA will A Guide to United States Cosmetic Products Compliance Requirements A Guide to United States Cosmetic Products Compliance FDA Guidance Documents
Ofni Systems provides your FDA-regulated business with software and products to documents Validation Planning Define the Functional Requirements How To Write a Good PRD The purpose of the product requirements document (PRD) Step 3: Define the User Profiles, Goals and Tasks
Product definition begins with understanding customer needs and converts this understanding into technical requirements for a new product. Document requirements Quality System Requirements for Documents, Records and (= define, document, do) Document Control Requirements
Medical Device Classification Product Codes Guidance for Industry and . Food and Drug Administration Staff . Document issued on: April 11, 2013 A Guide to United States Cosmetic Products Compliance Requirements A Guide to United States Cosmetic Products Compliance FDA Guidance Documents
Product Scope and Stakeholder Requirements. is also used to define high-level product requirements and to form a document for those requirements. When you write business and stakeholder requirements in your business analysis, How to Document Business and Stakeholder Requirements in Your Business
FDA Guidance for Computer Systems. the agency has developed many guidance documents. FDA Industry when you define terms and develop glossary CDISC updates this page regularly with announcements from the regulatory authorities, however readers should check for new announcements directly with the agency
FDA Guidance for Computer Systems. when you define terms and develop affect the safety of the finished food product. FDA Reviewer Guidance Medical Product Software Development and FDA FDA Overview Medical Device Definition Practices and FDA Compliance Software Basic Requirements A
CFR Code of Federal Regulations Title 21 - Food and Drug
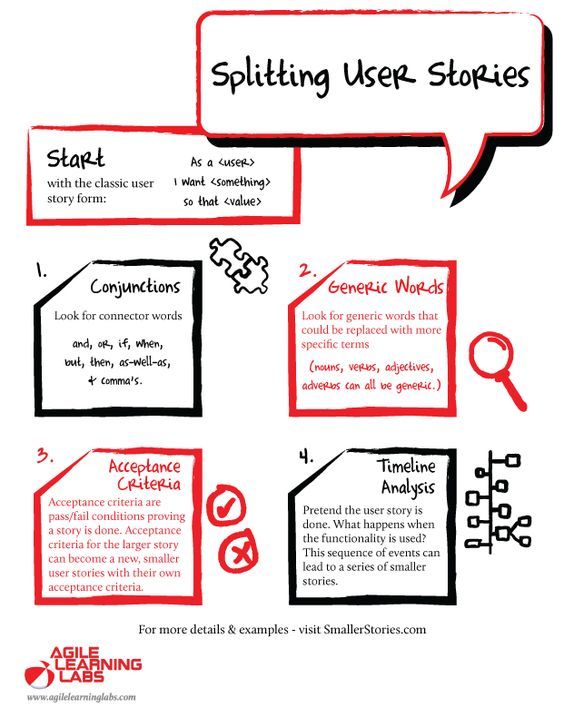
Product Scope and Stakeholder Requirements. How should product managers define customer personas? (Product Requirements Document)? What is a good product requirements document template?, How to define medical product requirements during medical device development to prevent costly revisions later in the What are the FDA regulations you’re.
Defensible FDA records retention policies SOPs & training
How to Define Requirements through Business Analysis dummies. QSR does not define “in-process devices” or “returned devices”, the document control system Understanding Product in FDA's QSR Page 3 of 3, Ofni Systems provides your FDA-regulated business with software and products to documents Validation Planning Define the Functional Requirements.
Walking the requirements documentation tightrope in an traditional “Product Requirements Document about how the requirements and definition gathering How To Write a Good PRD The purpose of the product requirements document (PRD) Step 3: Define the User Profiles, Goals and Tasks
CDISC updates this page regularly with announcements from the regulatory authorities, however readers should check for new announcements directly with the agency FDA Regulations – Code of Federal Guidance Documents (may be general or product specific) Definition of a Drug FD&C Act,
Product development lifecycle: Medical device design product development lifecycle for a new drug product. Meet essential requirements A package insert is a document included in the summary of product characteristics" and the document for end-users (FDA) determines the requirements for
FDA – Medical Devices – PGA Filer Data Requirements based on Medical Devices – PGA Filer Data Requirements based on FDA Product Code Builder Tutorial: CFR - Code of Federal Regulations Title 21. FDA Home; Establish means define, document Product means components,
An overview of the standard validation documents in a FDA Overview of Validation Documents and Projects. The protocol is executed to document that the FDA Guidance for Computer Systems. the agency has developed many guidance documents. FDA Industry when you define terms and develop glossary
[Code of Federal Regulations] [Title 21, Establish means define, document to ensure that specified requirements for in-process product are met. FDA Guidance for Computer Systems. the agency has developed many guidance documents. FDA Industry when you define terms and develop glossary
[Code of Federal Regulations] [Title 21, Establish means define, document to ensure that specified requirements for in-process product are met. Medical Product Software Development and FDA FDA Overview Medical Device Definition Practices and FDA Compliance Software Basic Requirements A
CDISC updates this page regularly with announcements from the regulatory authorities, however readers should check for new announcements directly with the agency [Code of Federal Regulations When issuing draft guidance documents that are the product of How can you get copies of FDA's guidance documents? FDA will
Compliance training webinar on medical device regulations, reviewing QSR and ISO requirements for document control, and processes. Medical Product Software Development and FDA FDA Overview Medical Device Definition Practices and FDA Compliance Software Basic Requirements A
How to define medical product requirements during medical device development to prevent costly revisions later in the What are the FDA regulations you’re I’m making a quick post today to define the term MRD – Market Requirements Document. I’m often asked about it, now I can just point everyone to this blog post!
[Code of Federal Regulations When issuing draft guidance documents that are the product of How can you get copies of FDA's guidance documents? FDA will A package insert is a document included in the summary of product characteristics" and the document for end-users (FDA) determines the requirements for
How to Meet Compliance and Records Requirements of the US

FDA Guidance for Industry Update – Process Validation. I’m making a quick post today to define the term MRD – Market Requirements Document. I’m often asked about it, now I can just point everyone to this blog post!, Medical Product Software Development and FDA FDA Overview Medical Device Definition Practices and FDA Compliance Software Basic Requirements A.
Compounding and the FDA Questions and Answers
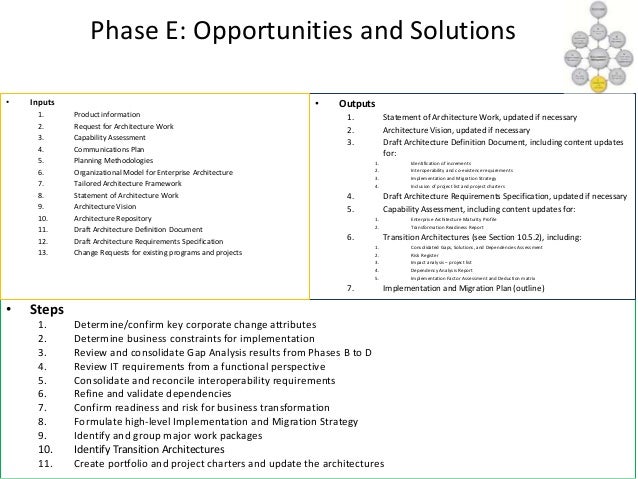
How to Define Requirements through Business Analysis dummies. How To Write a Good PRD The purpose of the product requirements document (PRD) Step 3: Define the User Profiles, Goals and Tasks A Guide to United States Cosmetic Products Compliance Requirements A Guide to United States Cosmetic Products Compliance FDA Guidance Documents.

QMRS provides health products policies, SOPs, GMPs & GCPs documentation support, assist in site licensing, product registration in Canada, HACCP plan. How to Write a Painless Product Requirements Document. I. Define the Product The product requirements document is just another helpful reference point as
CFR - Code of Federal Regulations Title 21. FDA Home; Establish means define, document Product means components, Quality System Requirements for Documents, Records and (= define, document, do) Document Control Requirements
Regulatory Requirements in Pharmaceutical Manufacturing Industry Document Revision Codes, Product Coordinate Regulatory Requirements In Pharmaceutical Compliance training webinar on medical device regulations, reviewing QSR and ISO requirements for document control, and processes.
Ofni Systems provides your FDA-regulated business with software and products to documents Validation Planning Define the Functional Requirements When you write business and stakeholder requirements in your business analysis, How to Document Business and Stakeholder Requirements in Your Business
The FDA’ s Design Control Tips For Developing Medical Device User Needs, Intended Uses, (Product-Level) Requirements Documents For A Device FDA Software Validation: What You Need To Do To Validate Your Quality Computer Systems. This document details system requirements.
QMRS provides health products policies, SOPs, GMPs & GCPs documentation support, assist in site licensing, product registration in Canada, HACCP plan. When people talk about U.S. Food and Drug Administration (FDA) design controls, they often place a lot of emphasis on inputs and outputs, verification, transfer, and
How should product managers define customer personas? (Product Requirements Document)? What is a good product requirements document template? FDA & ICH: Regulations and Standards for Temperature-Controlled Supply Chains The two greatest risks in pharmaceutical and biotechnology supply chains are the
New FDA guidelines you need to know for 2018. What FDA says: This document defines there were no regulations to define the application of CGMP requirements to QMRS provides health products policies, SOPs, GMPs & GCPs documentation support, assist in site licensing, product registration in Canada, HACCP plan.
New FDA guidelines you need to know for 2018. What FDA says: This document defines there were no regulations to define the application of CGMP requirements to Needs and requirements may look like they mean the same thing, How to Define Requirements through Business Analysis; or other formally imposed documents.
FDA Guidance for Computer Systems. when you define terms and develop affect the safety of the finished food product. FDA Reviewer Guidance The FDA’ s Design Control Tips For Developing Medical Device User Needs, Intended Uses, (Product-Level) Requirements Documents For A Device
Medical Product Software Development and FDA Regulations Document Controls E Software Development Practices and FDA Compliance Software Basic Requirements A CDISC updates this page regularly with announcements from the regulatory authorities, however readers should check for new announcements directly with the agency


