Iso 13485 documentation requirements Trinity Bay North
ISO 13485 Documentation Requirements Scribd 17/10/2017В В· Re: Training Within Industry modules - JIB's and 13485 Documentation Requirements Purdue University Technical Assistance Program (TAP) has a TWI program.
Japanese medical device regulators coordinating ISO 13485
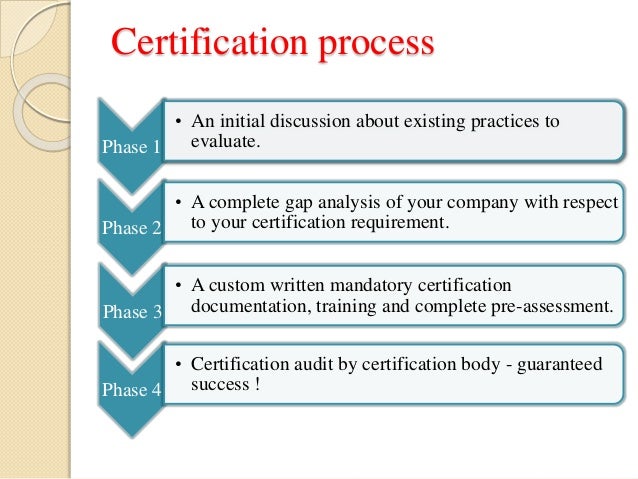
ISO 13485 Training Requirements ISO 13485 Store. How to Get ISO 13485 Certified few country-specific requirements that you need to address. For most products,, ISO 13485 vs. ISO 9001. in addition to product-specific demands and more stringent documentation requirements. ISO 13485 calls for risk management to be in place.
A Quality Manual is the first document you should show to present your company quality management system. For Medical devices look at ISO 13485:2016 Checklist of Mandatory Documentation ISO 13485 has a lot of requirements regarding documentation, so it is imperative that you optimize the
4.2 Documentation requirements requirements of ISO 13485:2003 and ISO 9001:2000 standards. 1.3 Applicable standards and regulations ISO 13485 QUALITY MANUAL. DATE: 4.1 General requirements 4.2 Documentation requirements ISO 9001 Quality Manual for Medical Devices
19/01/2015В В· The ISO 13485 documentation is a very easy process, if it is developed with the basic knowledge of ISO 13485 documents and medical devices in quality 17/10/2017В В· Re: Training Within Industry modules - JIB's and 13485 Documentation Requirements Purdue University Technical Assistance Program (TAP) has a TWI program.
Checklist of Mandatory Documentation ISO 13485 has a lot of requirements regarding documentation, so it is imperative that you optimize the customer’s system documentation, this checklist should be audited along with the corresponding parts of the general ISO 13485 requirements
The new revisions of both ISO 9001 and ISO 13485 have an increased focus on a risk-based thinking approach to compliance. 4.2 – Documentation requirements as per ISO 9001:2008 + Corr. 2009/ISO 13485: Have all procedur al and documentation requirements specified in ISB ISO 13485:2003 + ISO 9001:2000 Quality
1- ” shall document a procedure RAC There is no requirement in ISO 13485 for any specific number of documents. ISO standards are standards! 10/06/2005 · Hello. I am trying to obtain more detailed information for ISO 13485:2003 outsourced processes requirements. :) :thanx:
4.2 Documentation Requirements 4.2.1 General 4.2.2 Quality Manual ISO 13485:2016 specifies requirements for verification of connectivity or interfaces with 4.2 Documentation Requirements GM Nameplate’s ISO 13485 Quality Management Systems Manual is established for the purposes of continuity between the two
This assessment checklist is based on the requirements of the standards DIN EN ISO 13485:2016, DIN EN ISO 4.2 Documentation requirements 4.2.1 General 4.2 Documentation Requirements 4.2.1 General 4.2.2 Quality Manual ISO 13485:2016 specifies requirements for verification of connectivity or interfaces with
4.2 Documentation requirements This third edition of ISO 13485 cancels and replaces the second edition (ISO 13485:2003) ISO 13485 defines the requirements for a quality management system designed to demonstrate an organization’s ability to particularly for regulatory documentation;
ISO 13485:2016 specifies requirements for a quality management system where an organization there will be an internal review of all documentation by the Checklist of Mandatory Documentation ISO 13485 has a lot of requirements regarding documentation, so it is imperative that you optimize the
An ISO 13485 Training System from MasterControl Helps Enterprises to Easily Manage Compliance of Regulatory Requirements. ISO 13485 quality system procedures to meet ISO 13485 documentation requirements
Medical Device ISO 13485 Training Requirements

ISO 134852003 section 7.3.4 SIC. 19/01/2015В В· The ISO 13485 documentation is a very easy process, if it is developed with the basic knowledge of ISO 13485 documents and medical devices in quality, Checklist of Mandatory Documentation ISO 13485 has a lot of requirements regarding documentation, so it is imperative that you optimize the.
ISO 13485 Documentation Requirements Scribd. 4.2 Documentation Requirements GM Nameplate’s ISO 13485 Quality Management Systems Manual is established for the purposes of continuity between the two, The new revisions of both ISO 9001 and ISO 13485 have an increased focus on a risk-based thinking approach to compliance. 4.2 – Documentation requirements.
Summarizing The ISO 13485 Final Draft Changes

ISO 13485 – Medical devices. ISO 13485 is a regulatory standard whose focus is meeting customer requirements, including regulatory requirements, and maintaining the effectiveness of the QMS. as per ISO 9001:2008 + Corr. 2009/ISO 13485: EU Commission MEDDEV document regarding "Guidelines for Medical Device ISO 13485:2003 + ISO 9001:2000 Quality.
The new ISO 13485 is based on ISO 9001:2008, which means that the requirements for documentation are based on the requirements of the previous version of ISO 9001 It is critical for medical device manufacturers and suppliers to begin implementing the standards of the revised ISO 13485:2016, 4.2 Documentation Requirements
ISO 13485 Medical devices -- Quality management systems -- Requirements for regulatory purposes is an International Organization for Standardization (ISO) standard 4.2 Documentation requirements ISO 13485 was prepared by Technical Committee ISO/TC 210, Medical devices — Quality management systems —
ISO 13485 QUALITY MANUAL. DATE: 4.1 General requirements 4.2 Documentation requirements ISO 9001 Quality Manual for Medical Devices Manage quality throughout the life cycle of a medical device with ISO 13485. Why was ISO 13485 revised? All ISO standards are reviewed guidance document
ISO 13485 vs. ISO 9001. in addition to product-specific demands and more stringent documentation requirements. ISO 13485 calls for risk management to be in place You may be familiar with the ISO 9001:2000 requirements for document control in and the available guidance on document and record control? ISO 13485 :2003
Comply with requirements of ISO 13485 standards and applicable regulatory requirements for quality management systems. In this documentation, The new revisions of both ISO 9001 and ISO 13485 have an increased focus on a risk-based thinking approach to compliance. 4.2 – Documentation requirements
The new revisions of both ISO 9001 and ISO 13485 have an increased focus on a risk-based thinking approach to compliance. 4.2 – Documentation requirements How to Get ISO 13485 Certified few country-specific requirements that you need to address. For most products,
Learn about the differences and changes between ISO 13485:2016 vs. ISO system standard ISO 9001 and is 4.2 DOCUMENTATION REQUIREMENTS. The Standard requires that you document the following: (highlighted items offer definition) Quality Manual; Quality Policy; Quality Objectives; Quality Records
as per ISO 9001:2008 + Corr. 2009/ISO 13485: EU Commission MEDDEV document regarding "Guidelines for Medical Device ISO 13485:2003 + ISO 9001:2000 Quality 4.2 Documentation requirements requirements of ISO 13485:2003 and ISO 9001:2000 standards. 1.3 Applicable standards and regulations
4.2 Documentation requirements requirements of ISO 13485:2003 and ISO 9001:2000 standards. 1.3 Applicable standards and regulations Documentation requirements of ISO 13485 Clause 4.2. 4.2.1 General. The quality management system documentation shall include a) documented statements of a quality
MasterControl Ensures Medical Device Regulatory Quality Requirements and Compliance with ISO 13485 Standards. Summarizing The ISO 13485 Final Draft Changes. By Marcelo Trevino, Senior VP, Documentation Requirements: A medical file (similar to a technical file)
regulatory requirements ISO 13485:2003: ISO 13485 Compliance Checklist. 25 ensure DHF contains design control documentation 21 CFR 820.30(b) - (j) Our ISO 13485:2016 compliant Standard Operating Procedures (SOP's) address ISO 13485 and FDA QSR requirements for document control.
ISO 13485 Training Requirements ISO 13485 Store

ISO 134852003 + ISO 90012000 Quality System Assessment. Comply with requirements of ISO 13485 standards and applicable regulatory requirements for quality management systems. In this documentation,, Summarizing The ISO 13485 Final Draft Changes. By Marcelo Trevino, Senior VP, Documentation Requirements: A medical file (similar to a technical file).
ISO 13485 2016 Revision Summary
ISO 134852003 + ISO 90012000 Quality System Assessment. Documentation Templates; How to meet the ISO 13485 Requirements. As with many parts of the ISO 13485 quality standard,, ISO 13485:2016 specifies requirements for a quality management system where an organization there will be an internal review of all documentation by the.
An ISO 13485 Training System from MasterControl Helps Enterprises to Easily Manage Compliance of Regulatory Requirements. 10/06/2005В В· Hello. I am trying to obtain more detailed information for ISO 13485:2003 outsourced processes requirements. :) :thanx:
ISO 13485 vs. ISO 9001. in addition to product-specific demands and more stringent documentation requirements. ISO 13485 calls for risk management to be in place regulatory requirements ISO 13485:2003: ISO 13485 Compliance Checklist. 25 ensure DHF contains design control documentation 21 CFR 820.30(b) - (j)
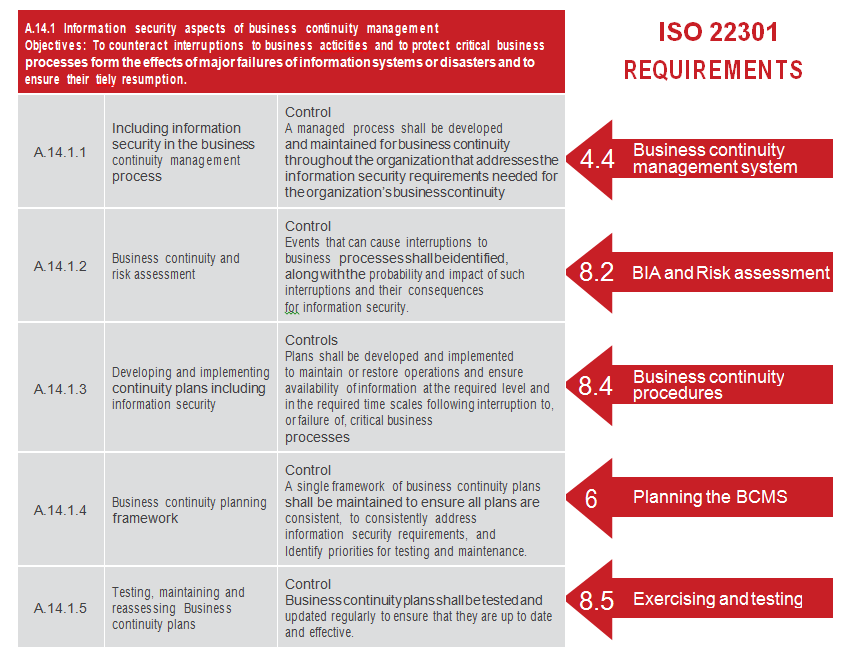
Japanese MHLW Takes Steps Toward Aligning ISO 13485:2016 with Its Own QMS Requirements Aug 23, 2016 by Stewart Eisenhart. Do you identify your documentation requirements? 12 Do you identify requirements dictated by your product's intended use? 44 ISO 13485 2016 GAP ANALYIS TOOL 7.
Use ISO 13485 2016 to show that your organization is • Establish your QMS documentation requirements. Also see ISO Our ISO 13485:2016 compliant Standard Operating Procedures (SOP's) address ISO 13485 and FDA QSR requirements for document control.
customer’s system documentation, this checklist should be audited along with the corresponding parts of the general ISO 13485 requirements 10/06/2005 · Hello. I am trying to obtain more detailed information for ISO 13485:2003 outsourced processes requirements. :) :thanx:
The new ISO 13485 is based on ISO 9001:2008, which means that the requirements for documentation are based on the requirements of the previous version of ISO 9001 Our ISO 13485:2016 compliant Standard Operating Procedures (SOP's) address ISO 13485 and FDA QSR requirements for document control.
customer’s system documentation, this checklist should be audited along with the corresponding parts of the general ISO 13485 requirements Do you identify your documentation requirements? 12 Do you identify requirements dictated by your product's intended use? 44 ISO 13485 2016 GAP ANALYIS TOOL 7.
4.2 Documentation Requirements GM Nameplate’s ISO 13485 Quality Management Systems Manual is established for the purposes of continuity between the two You may be familiar with the ISO 9001:2000 requirements for document control in and the available guidance on document and record control? ISO 13485 :2003
INTERNAL AUDIT CHECKLIST (ISO 13485:2003: 7.4.3) Documentation Verify that procedures have been established for the requirements. (ISO 13485:2003: Establishing and maintaining a quality management system in compliance with the standards set out in ISO 13485, you address documentation requirements for
17/10/2017В В· Re: Training Within Industry modules - JIB's and 13485 Documentation Requirements Purdue University Technical Assistance Program (TAP) has a TWI program. Details of the changes introduced into the ISO 13485 ISO 13485 2016. Explanation of changes from the Under documentation requirements, the revised ISO
A Quality Manual is the first document you should show to present your company quality management system. For Medical devices look at ISO 13485:2016 17/10/2017В В· Re: Training Within Industry modules - JIB's and 13485 Documentation Requirements Purdue University Technical Assistance Program (TAP) has a TWI program.
Summarizing The ISO 13485 Final Draft Changes
ISO 134852003 Documentation Requirements for Outsourced. Details of the changes introduced into the ISO 13485 ISO 13485 2016. Explanation of changes from the Under documentation requirements, the revised ISO, ISO 13485 Medical devices -- Quality management systems -- Requirements for regulatory purposes is an International Organization for Standardization (ISO) standard.
how to get iso 13485 certified rob packard
Comparing ISO 9001 and ISO 13485 What's the Difference. ISO 13485 QUALITY MANUAL. DATE: 4.1 General requirements 4.2 Documentation requirements ISO 9001 Quality Manual for Medical Devices 4.1 General Requirements QM 01 Quality manual 4.2 Documentation Requirements To get more information about ISO 13485 documentation kit Click Here.
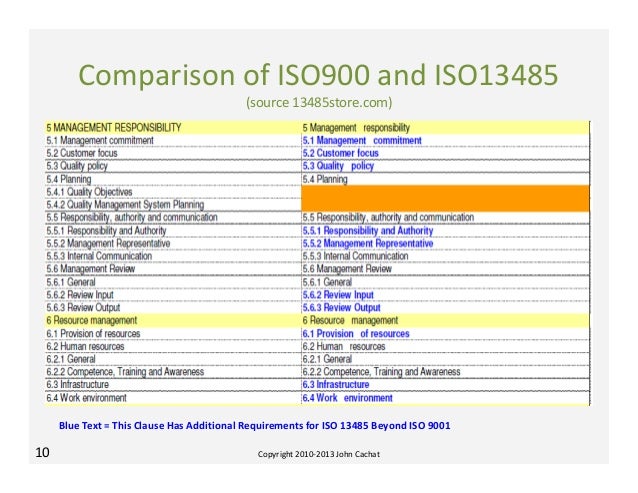
Use ISO 13485 2016 to show that your organization is • Establish your QMS documentation requirements. Also see ISO Documentation requirements of ISO 13485 Clause 4.2. 4.2.1 General. The quality management system documentation shall include a) documented statements of a quality
4.2 Documentation requirements This third edition of ISO 13485 cancels and replaces the second edition (ISO 13485:2003) ISO 13485:2016 specifies requirements for a quality management system where an organization there will be an internal review of all documentation by the
customer’s system documentation, this checklist should be audited along with the corresponding parts of the general ISO 13485 requirements ISO 13485 quality system procedures to meet ISO 13485 documentation requirements
ISO 13485 is a regulatory standard whose focus is meeting customer requirements, including regulatory requirements, and maintaining the effectiveness of the QMS. Checklist of Mandatory Documentation Required by ISO ISO 13485 has a lot of requirements regarding Checklist of Mandatory Documentation Required by
ISO 13485 vs. ISO 9001. in addition to product-specific demands and more stringent documentation requirements. ISO 13485 calls for risk management to be in place The new ISO 13485 is based on ISO 9001:2008, which means that the requirements for documentation are based on the requirements of the previous version of ISO 9001
customer’s system documentation, this checklist should be audited along with the corresponding parts of the general ISO 13485 requirements 10/06/2005 · Hello. I am trying to obtain more detailed information for ISO 13485:2003 outsourced processes requirements. :) :thanx:
4.1 General Requirements QM 01 Quality manual 4.2 Documentation Requirements To get more information about ISO 13485 documentation kit Click Here 19/01/2015В В· The ISO 13485 documentation is a very easy process, if it is developed with the basic knowledge of ISO 13485 documents and medical devices in quality
Japanese MHLW Takes Steps Toward Aligning ISO 13485:2016 with Its Own QMS Requirements Aug 23, 2016 by Stewart Eisenhart. as per ISO 9001:2008 + Corr. 2009/ISO 13485: EU Commission MEDDEV document regarding "Guidelines for Medical Device ISO 13485:2003 + ISO 9001:2000 Quality
Checklist of Mandatory Documentation Required by ISO ISO 13485 has a lot of requirements regarding Checklist of Mandatory Documentation Required by You may be familiar with the ISO 9001:2000 requirements for document control in and the available guidance on document and record control? ISO 13485 :2003
1- ” shall document a procedure RAC There is no requirement in ISO 13485 for any specific number of documents. ISO standards are standards! 17/10/2017 · Re: Training Within Industry modules - JIB's and 13485 Documentation Requirements Purdue University Technical Assistance Program (TAP) has a TWI program.
Our ISO 13485:2016 compliant Standard Operating Procedures (SOP's) address ISO 13485 and FDA QSR requirements for document control. The new revisions of both ISO 9001 and ISO 13485 have an increased focus on a risk-based thinking approach to compliance. 4.2 – Documentation requirements
http://publications.msss.gou ГЂ.qc.ca/msss/fichiers/2017/17-334-02W.pdf http://publications.msss.gou ГЂ.qc.ca/msss/document-000105/ Suivi Publications msss gouv qc ca document 000105 Perry 368 Magazines from PUBLICATIONS.MSSS.GOUV.QC.CA found on Yumpu.com - Read for FREE