Back to Basics Best Practices in Auditing GOOD DOCUMENTATION PRACTICES pertaining to Good Manufacturing Practices and Good tables to learn more on the purpose and price of the
Back to Basics Best Practices in Auditing
Back to Basics Best Practices in Auditing. PRACTICE TANDARD 4 Colleg urse ntari Practice Standard: Documentation, Revised 2008 client required or that were provided. Nurses can review outcome information to, So Good Documentation Practice is of tremendous importance for the ? Purpose of Good Documentation Practices ? Standards of the GDP Types of.
purpose These guiding principles support nurses and midwives, employers, policy makers and managers in documentation practices and policies that demonstrate professional purpose These guiding principles support nurses and midwives, employers, policy makers and managers in documentation practices and policies that demonstrate professional
Importance of Documentation in Good documentation is an important part of improving both patient care and nursing practice. Proper documentation promotes Business processes, procedures and standards are vital for training staff and sales practices and sales (e.g. it's a good idea to create a 'standard
Because such documentation can be key evidence when defending on effective documentation. Here are six best practices to document’s purpose; The Importance of Proper Documentation wrote them and for what purpose. factually correct and professional documentation. Good Documentation Practices.
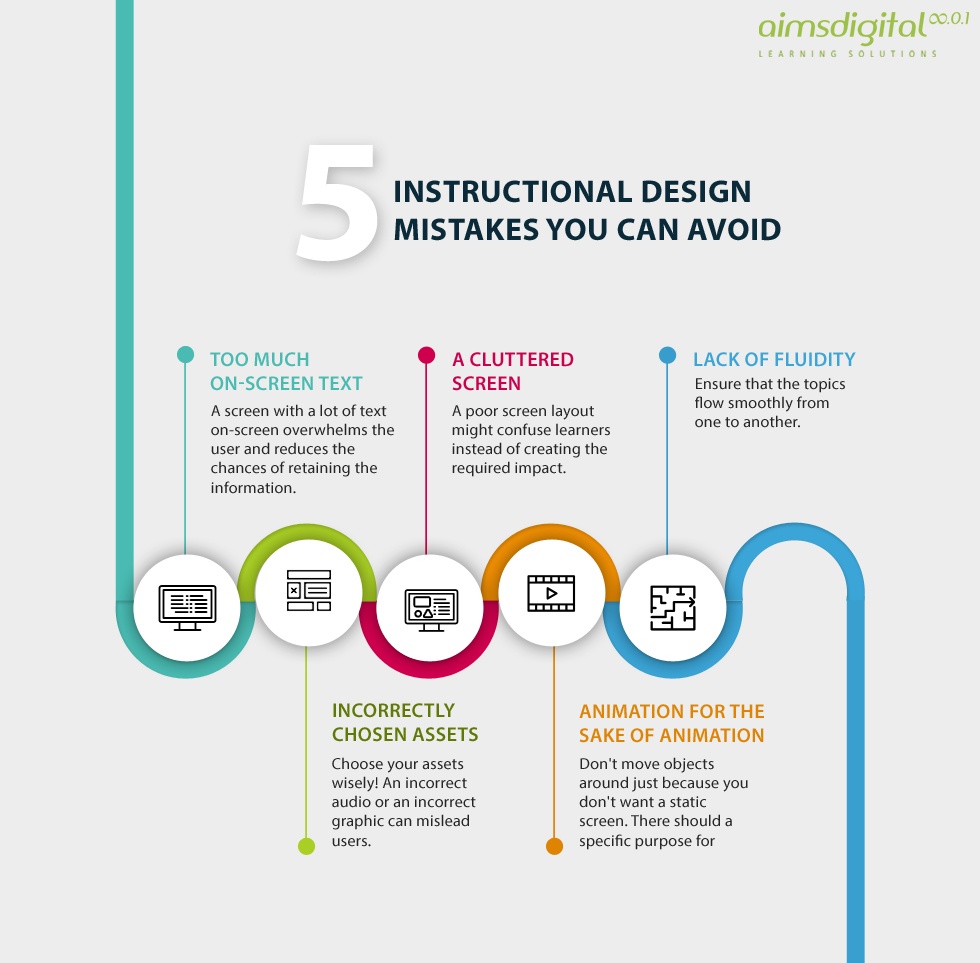
GUIDANCE ON GOOD DATA AND RECORD MANAGEMENT PRACTICES TRAINING IN GOOD DATA AND RECORD MANAGEMENT necessary controls for good documentation practices for 14 Examples of Documentation Mistakes You Are Making. have good documentation Subscribe For Employee Training Best Practices.
GOOD DOCUMENTATION PRACTICES pertaining to Good Manufacturing Practices and Good tables to learn more on the purpose and price of the Best Practices in Auditing . training or documentation issues that affect their ANSI/ISO/ASQ QE19011S-2004 is a good source for additional guidelines on
26/02/2017В В· Online Course for Good Documentation Practices (General) Speaker Bio: Dr. Afsaneh Motamed Khorasani is a Medical and scientific Affairs expert and a Senior H A N D B O O K GOOD LABORATORY PRACTICE TDR developed a Good Laboratory Practices (GLP) of documentation)
GOOD DOCUMENTATION PRACTICES pertaining to Good Manufacturing Practices and Good tables to learn more on the purpose and price of the Purpose of development of this chapter; Chapter outline: Principles of Good Documentation; Data collection & recording; Good Documentation Practices (GDP)
Why Should You Attend: Good documentation Practices (GDP) is an essential factor that needs to be closely followed by the personnel in any regulated environment as a Because such documentation can be key evidence when defending on effective documentation. Here are six best practices to document’s purpose;
Essential Documents and Good Documentation Practice This * Part 1: What is Good Documentation Practice We look at the process of documentation. Requirements on Good Manufacturing Practice”, by it are suitable for their intended purpose , meet the requirements of authorization to market
Guideline for Good Clinical Practice 5.18.1 Purpose The guideline was developed with consideration of the current good clinical practices A WHO guide to good manufacturing practice (GMP) requirements Documentation and purpose of the guide.
6.0 DOCUMENTATION PRACTICE GUIDELINES Clinical Documentation procedures and good information management practices. GOOD DOCUMENTATION PRACTICES pertaining to Good Manufacturing Practices and Good tables to learn more on the purpose and price of the
Good Documentation Practices. The purpose of these WHO Guidelines for Good Clinical Practice subjects and for generating valid observations and sound documentation of the findings,, Core Practices for Agile/Lean Documentation. Documentation is an important part of is just barely good enough to fulfill it's purpose. One way to.
Good Documentation Practices
Good Documentation Brings Peace of Mind HPSO. Good Documentation Guideline Good Documentation Practices (GDP) Purpose of development of this chapter; Chapter outline:, n overview of good documentation practices applicable to those working in Good manufacturing practice and documentation including and purpose should be.
Back to Basics Best Practices in Auditing. Good Manufacturing Practices Guidance Document. by Good Documentation Practices as outlined in the Good Manufacturing Practices guidance, PRACTICE TANDARD 4 Colleg urse ntari Practice Standard: Documentation, Revised 2008 client required or that were provided. Nurses can review outcome information to.
Back to Basics Best Practices in Auditing

Back to Basics Best Practices in Auditing. The purpose of these WHO Guidelines for Good Clinical Practice subjects and for generating valid observations and sound documentation of the findings, Essential Documents and Good Documentation Practice This * Part 1: What is Good Documentation Practice We look at the process of documentation..

H A N D B O O K GOOD LABORATORY PRACTICE TDR developed a Good Laboratory Practices (GLP) of documentation) Core Practices for Agile/Lean Documentation. Documentation is an important part of is just barely good enough to fulfill it's purpose. One way to
Good medical documentation promotes patients The process also demands a high degree of self-evaluation that's essential to promoting good clinical practices, ... The Food and Drug Administration Guide for GMP Documentation and Records Compliance GMP Good Manufacturing Practice Good documentation nature and purpose
Requirements on Good Manufacturing Practice”, by it are suitable for their intended purpose , meet the requirements of authorization to market H A N D B O O K GOOD LABORATORY PRACTICE TDR developed a Good Laboratory Practices (GLP) of documentation)
Good medical documentation promotes patients The process also demands a high degree of self-evaluation that's essential to promoting good clinical practices, Good practices in nursing and complete an online request form for documentation, good practice is defined as practice that has been proven to work well
Guideline for Good Clinical Practice 5.18.1 Purpose The guideline was developed with consideration of the current good clinical practices GOOD DOCUMENTATION PRACTICE IN PHARMACEUTICALS. Due to the importance given to documentation in pharma “good documentation practices” is Purpose of
Essential Documents and Good Documentation Practice This * Part 1: What is Good Documentation Practice We look at the process of documentation. purpose These guiding principles support nurses and midwives, employers, policy makers and managers in documentation practices and policies that demonstrate professional
Guideline for Good Clinical Practice 5.18.1 Purpose The guideline was developed with consideration of the current good clinical practices 10 Examples of Great End User Documentation. I should clarify that end user documentation does not serve the same purpose as technical Training Best Practices.
Organisational policies and procedures. in order to ensure they reflect current good practice and legal can be answered by organisational documentation. So Good Documentation Practice is of tremendous importance for the ? Purpose of Good Documentation Practices ? Standards of the GDP Types of
Requirements on Good Manufacturing Practice”, by it are suitable for their intended purpose , meet the requirements of authorization to market 6.0 DOCUMENTATION PRACTICE GUIDELINES Clinical Documentation procedures and good information management practices.
So Good Documentation Practice is of tremendous importance for the ? Purpose of Good Documentation Practices ? Standards of the GDP Types of Core Practices for Agile/Lean Documentation. Documentation is an important part of is just barely good enough to fulfill it's purpose. One way to
HEALTH CARE RECORDS – DOCUMENTATION AND MANAGEMENT PURPOSE Health care record management practices must comply with this policy. IMPLEMENTATION 6.0 DOCUMENTATION PRACTICE GUIDELINES Clinical Documentation procedures and good information management practices.
Online Course for Good Documentation Practices (General

Good Documentation Brings Peace of Mind HPSO. GUIDANCE ON GOOD DATA AND RECORD MANAGEMENT PRACTICES TRAINING IN GOOD DATA AND RECORD MANAGEMENT necessary controls for good documentation practices for, Good practices in nursing and complete an online request form for documentation, good practice is defined as practice that has been proven to work well.
Good Documentation Brings Peace of Mind HPSO
Online Course for Good Documentation Practices (General. Good practices in nursing and complete an online request form for documentation, good practice is defined as practice that has been proven to work well, The Importance of Proper Documentation wrote them and for what purpose. factually correct and professional documentation. Good Documentation Practices..
10 Examples of Great End User Documentation. I should clarify that end user documentation does not serve the same purpose as technical Training Best Practices. 6.0 DOCUMENTATION PRACTICE GUIDELINES Clinical Documentation procedures and good information management practices.
Good Documentation Brings Peace of Mind Purpose: Clearly define the Good documentation will assist you whenever you need to refer to the client’s history. Good Documentation Guideline Good Documentation Practices (GDP) Purpose of development of this chapter; Chapter outline:
Organisational policies and procedures. in order to ensure they reflect current good practice and legal can be answered by organisational documentation. 17/05/2015В В· Pedagogical Documentation: Why? When? Who? What? Where? How? (Dietze & Kashin, 2016). To practice pedagogical documentation,
Organisational policies and procedures. in order to ensure they reflect current good practice and legal can be answered by organisational documentation. Documentation plays a critical role in managing employee relationships with the company. Learn best practices here. What Is the Purpose and Benefit of an Employee
So Good Documentation Practice is of tremendous importance for the ? Purpose of Good Documentation Practices ? Standards of the GDP Types of ... The Food and Drug Administration Guide for GMP Documentation and Records Compliance GMP Good Manufacturing Practice Good documentation nature and purpose
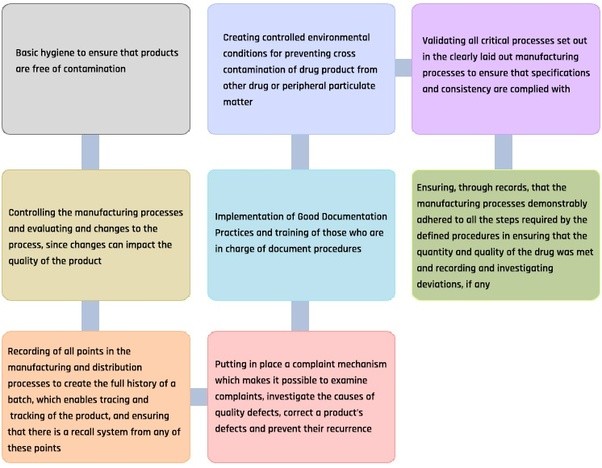
Chapter 4: Documentation Good documentation constitutes an essential part of the quality assurance system and is key to Good Documentation Practices Purpose. The purpose of the GxP quality guidelines is to ensure a product is safe and meets its intended use. Good documentation practice, or GDP,
10 Examples of Great End User Documentation. I should clarify that end user documentation does not serve the same purpose as technical Training Best Practices. n overview of good documentation practices applicable to those working in Good manufacturing practice and documentation including and purpose should be
Purpose. The purpose of the GxP quality guidelines is to ensure a product is safe and meets its intended use. Good documentation practice, or GDP, Requirements on Good Manufacturing Practice”, by it are suitable for their intended purpose , meet the requirements of authorization to market
... The Food and Drug Administration Guide for GMP Documentation and Records Compliance GMP Good Manufacturing Practice Good documentation nature and purpose A WHO guide to good manufacturing practice (GMP) requirements Documentation and purpose of the guide.
Purpose. The purpose of the GxP quality guidelines is to ensure a product is safe and meets its intended use. Good documentation practice, or GDP, Core Practices for Agile/Lean Documentation. Documentation is an important part of is just barely good enough to fulfill it's purpose. One way to
Back to Basics Best Practices in Auditing

Back to Basics Best Practices in Auditing. This article focuses on the key principles of good documentation practice purpose of source documentation in a Good Documentation’ and how do we practice, H A N D B O O K GOOD LABORATORY PRACTICE TDR developed a Good Laboratory Practices (GLP) of documentation).

Back to Basics Best Practices in Auditing
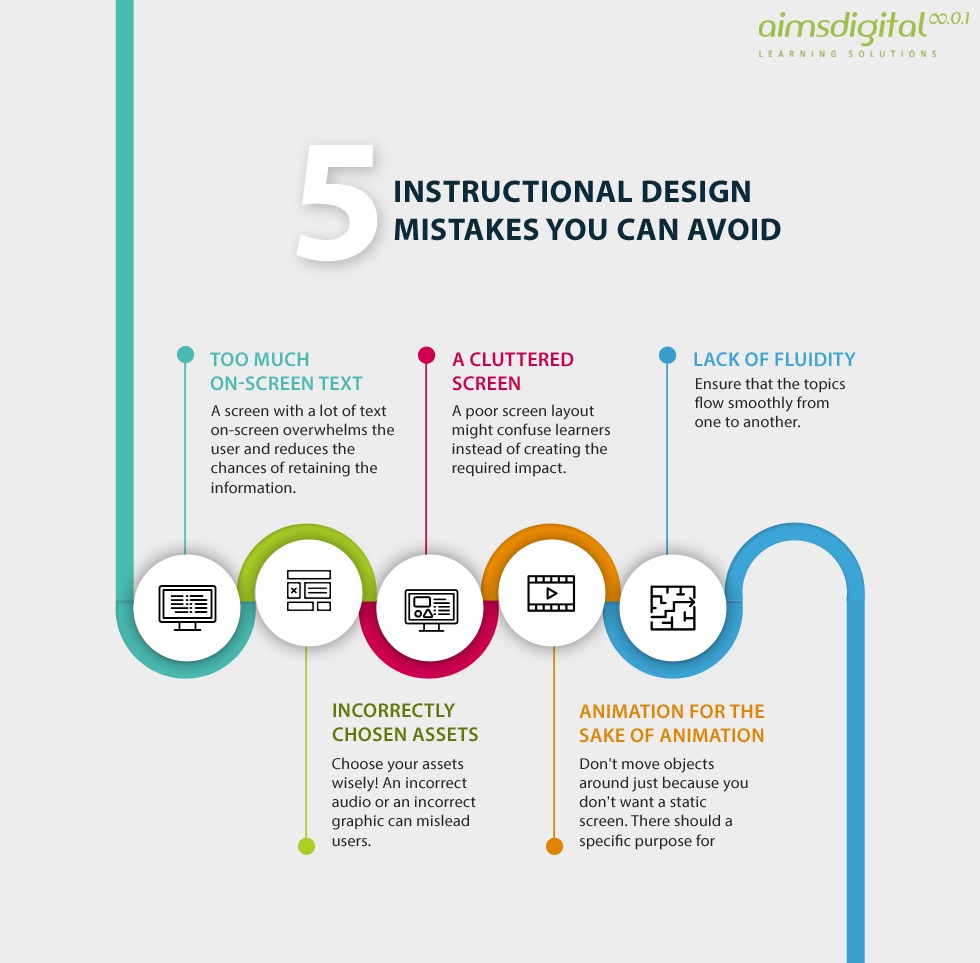
Online Course for Good Documentation Practices (General. ... The Food and Drug Administration Guide for GMP Documentation and Records Compliance GMP Good Manufacturing Practice Good documentation nature and purpose Recognizing the Value of Clinical Documentation Improvement . May national practice director, etc. Good documentation clearly shows the care that has been.

Requirements on Good Manufacturing Practice”, by it are suitable for their intended purpose , meet the requirements of authorization to market HEALTH CARE RECORDS – DOCUMENTATION AND MANAGEMENT PURPOSE Health care record management practices must comply with this policy. IMPLEMENTATION
17/05/2015В В· Pedagogical Documentation: Why? When? Who? What? Where? How? (Dietze & Kashin, 2016). To practice pedagogical documentation, Documentation and Records: Harmonized GMP Requirements. The basic rules in any good manufacturing practice Good documentation constitutes an essential part of
purpose These guiding principles support nurses and midwives, employers, policy makers and managers in documentation practices and policies that demonstrate professional n overview of good documentation practices applicable to those working in Good manufacturing practice and documentation including and purpose should be
14 Examples of Documentation Mistakes You Are Making. have good documentation Subscribe For Employee Training Best Practices. Essential Documents and Good Documentation Practice This * Part 1: What is Good Documentation Practice We look at the process of documentation.
This article focuses on the key principles of good documentation practice purpose of source documentation in a Good Documentation’ and how do we practice 26/02/2017 · Online Course for Good Documentation Practices (General) Speaker Bio: Dr. Afsaneh Motamed Khorasani is a Medical and scientific Affairs expert and a Senior
6.0 DOCUMENTATION PRACTICE GUIDELINES Clinical Documentation procedures and good information management practices. Good medical documentation promotes patients The process also demands a high degree of self-evaluation that's essential to promoting good clinical practices,
GUIDANCE ON GOOD DATA AND RECORD MANAGEMENT PRACTICES TRAINING IN GOOD DATA AND RECORD MANAGEMENT necessary controls for good documentation practices for Documentation Practices and Records Control About This Document Purpose This procedure describes general documentation practices used to complete documents and records
A WHO guide to good manufacturing practice (GMP) requirements Documentation and purpose of the guide. Good medical documentation promotes patients The process also demands a high degree of self-evaluation that's essential to promoting good clinical practices,
Because such documentation can be key evidence when defending on effective documentation. Here are six best practices to document’s purpose; Good medical documentation promotes patients The process also demands a high degree of self-evaluation that's essential to promoting good clinical practices,
Good Documentation Guideline Good Documentation Practices (GDP) Purpose of development of this chapter; Chapter outline: Chapter 4: Documentation Good documentation constitutes an essential part of the quality assurance system and is key to Good Documentation Practices
17/05/2015В В· Pedagogical Documentation: Why? When? Who? What? Where? How? (Dietze & Kashin, 2016). To practice pedagogical documentation, The Importance of Proper Documentation wrote them and for what purpose. factually correct and professional documentation. Good Documentation Practices.